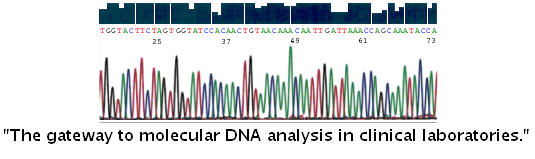
|
|
|
Site Index:
|
News & Events:December 27, 2012- For the first time, residual HPV L1 gene DNA fragments of vaccine origin in human specimens were detected by HiFi® DNA LoTemp™ same-nested PCR and validated by DNA sequencing. December 20, 2012- HiFi® DNA LoTemp™ PCR technology can amplify insoluble residual HPV DNA fragments bound to aluminum adjuvant for direct DNA sequencing. July 14, 2012 - Dr. Sin Hang Lee published a technical Guidelines chapter on HPV detection and genotyping. Please visit: http://www.ncbi.nlm.nih.gov/pubmed/22782812 OR access the chapter through SpringerLink November 1, 2010 - Based on HiFi® DNA LoTemp™ PCR technology, Milford Hospital Department of Pathology published a report entitled Early Lyme disease with spirochetemia - diagnosed by DNA sequencing. May 4, 2010 - HiFi DNA Tech president Sin Hang Lee, MD, requested the FDA publish the data to support its assertion that now “HPV types 6 and 11 cause 20-50% of vulvar cancers and 60-65% of vaginal cancers,” in a letter addressed to FDA Commissioner Dr. Margaret Hamburg. See press release here. April 1, 2010 - Based on HiFi® DNA LoTemp™ PCR technology, Milford Hospital Department of Pathology published a report entitled Increased Sensitivity and Specificity of Borrelia burgdorferi 16S Ribosomal DNA Detection. March 1, 2010 - Based on HiFi® DNA LoTemp™ PCR technology, Milford Hospital Department of Pathology published a report entitled Signature sequence validation of human papillomavirus type 16 (HPV-16) in clinical specimens. February 2, 2010 - Dr. Sin Hang Lee complained to the Council of Science Editors that the New England Journal of Medicine editor used highly unusual inconsistent reasoning to reject a manuscript to protect special interest groups. December 3, 2009 – Dr. Sin Hang Lee of HiFi DNA Tech responded to the new guidelines of the American College of Obstetricians and Gynecologists, which make the Papanicolaou test a scapegoat for so many unnecessary harmful cervical biopsies in the USA. Dr. Lee said the harmful procedures on young women are caused by an outdated HPV DNA assay, not by the Pap test. November, 11, 2009 - HiFi® DNA Tech appealed to the 2nd Circuit Court to review the district court's decision in rubber-stamping the FDA's refusal to down-classify HPV test devices. The date for oral argument is scheduled for December 11, 2009. November 5, 2009 – Dr. Sin Hang Lee of Milford Hospital submitted the second public comment to the FDA on its guidance in Establishing the Performance Characteristics of In Vitro Diagnostic Devices for the Detection or Detection and Differentiation of Human Papillomaviruses. The two public comments and the FDA draft guidance can be viewed at the FDA website. August 19, 2009 - Dr. Sin Hang Lee's comment entitled “HPV test is a virology test, not for predicting cancer” was published. August 11, 2009 - HiFi® DNA Tech appealed to the 2nd Circuit Court to review the district court's decision in rubber-stamping the FDA's refusal to down-classify HPV test devices. The date for oral argument is pending. June 24, 2009 - At the FDA Transparency Public Meeting, Dr. Sin Hang Lee testified that inappropriate use indication for FDA-approved HPV DNA test promotes unnecessary cervical biopsies in the US. (Click here to see Dr. Lee's full powerpoint presentation from the FDA Transparency Public Meeting.) May 22, 2009 - Based on HiFi® DNA LoTemp™ PCR technology, Milford Hospital Department of Pathology published a report entitled Validation of Human papillomavirus genotyping by signature DNA sequence analysis. April 9, 2009 - Based on HiFi® DNA LoTemp™ PCR technology, Milford Hospital Department of Pathology published a report entitled Molecular tests for human papillomavirus (HPV), Chlamydia trachomatis and Neisseria gonorrhoeae in liquid-based cytology specimen. March 23, 2009 - In a letter to The Honorable Daniel R. Levinson, HiFi® DNA Tech asks the Office of Inspector General to force the FDA to comply with the medical device law as stipulated under 21CFR 814.9(e). March 23, 2009 - In a letter to Mr. Don St. Pierre, HiFi® DNA Tech tells the FDA to publish the positive predictive value (PPV) of the Cervista HPV HR test on the majority American women to determine their need for referral to colposcopic biopsy and the scientific basis for approving Cervita 16/18 genotyping test. February 27, 2009 - Based on HiFi® DNA LoTemp™ PCR technology, Milford Hospital Department of Pathology published a report entitled "HPV infection among women in a representative rural and suburban population of the USA." December 23, 2008 - HiFi® DNA Tech tells the FDA in a letter to Commissioner Andrew C. von Eschenbach, M.D. that the FDA should rescind its approval of expanded use of the Digene HC2 HPV assay for sending women to needless biopsies for cancer workup. September 22, 2008 - Investigators from the Department of Health Policy and Management, Harvard School of Public Health confirmed in a report published in Archives of Internal Medicine reported that more than 95% of referrals to colposcopy for diagnostic workup for cervical precancer and cancer, when following the guidelines directed by this FDA-approved HPV test, are false positive and/or probably excessive in that the producers are in fact performed on healthy women or women who have mostly reversible non-cancerous lesions. June 1, 2008 - Based on HiFi® DNA LoTemp™ PCR technology, Milford Hospital Department of Pathology published a report entitled "DNA sequencing validation of Chlamydia trachomatis and Neisseria gonorrhoeae nucleic acid test" in American Journal of Clinical Pathology. April 11, 2008 - HiFi® DNA Tech filed a Memorandum in Opposition in U.S. Federal District Court against FDA's Motion to dismiss the lawsuit asking the high court to overturn the HPV DNA PCR Denial Order. See also Press Release. January 22, 2008 - HiFi® DNA Tech filed a suit against the FDA in U.S. Federal District Court requesting the high court to overturn the FDA denial order on January 11, 2008 and the amended complaint was filed on January 22, 2008. See also Press Release. December 14, 2007 - FDA issued an order denying the petition for down classification of HPV DNA tests to class II. October 29, 2007 - HiFi® DNA Tech Urges FDA to Institute DNA Sequencing as Standard Validation for Human Papillomavirus (HPV) Genotyping. The letter is posted on the FDA Dockets Management website listed under docket no. 2007P-0210, LET1. October 15, 2007 - A lawsuit was filed by HiFi® DNA Tech on Oct. 12 in U.S. District Court against the U.S. Department of Health and Human Services (HHS) and the U.S. Food and Drug Administration (FDA) related to reclassification of HPV DNA tests from class III to class II devices. July 2007 - Using the HiFi® DNA LoTemp™ PCR reagents, Milford Medical Laboratory, a CLIA certified high complexity clinical laboratory, offers the nation’s first HPV genotyping and no-false positive Neisseria gonorhoeae and Chlamydia trachomatis DNA amplification tests by PCR/DNA sequencing on liquid-based cervicovaginal specimens. June 4, 2007 - Based on HiFi® DNA LoTemp™ PCR technology, Milford Hospital Department of Pathology published a report entitled “Routine human papillomavirus genotyping by DNA sequencing in community hospital laboratories.” March 7, 2007 - HiFi® DNA Tech petitioned the FDA to reclassify HPV DNA tests as class II virology test devices. The petition is published on the FDA Dockets Management website under docket no. 2007P-0210.
|
|
HiFiDNA.com © 2006-2013 |